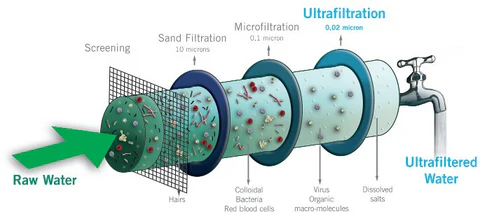
Ultrafiltration is one of those phrases that sounds technical but really describes a simple physical idea: pushing liquid through a fine filter while leaving larger things (like proteins or cells) behind. In the body, ultrafiltration is most important in the kidney’s glomerulus, where blood is filtered to form the first stage of urine. It’s also relevant in capillary exchange everywhere, and is the principle behind fluid removal in dialysis. Below I’ll explain anatomy, the driving forces, the key molecules, how the process is regulated, and why it matters clinically — all in plain language but with enough depth for medical students.
What exactly is being filtered?
In the glomerulus we separate plasma water and small dissolved solutes (electrolytes, glucose, amino acids, small peptides) from the cells and most plasma proteins. That filtered water and solutes enter Bowman’s space as glomerular filtrate. The cells (red cells, white cells) and larger proteins normally stay in the capillary.
Think of the glomerulus as a coffee maker: water and soluble taste compounds pass through the filter paper, but the grounds (cells, big proteins) stay behind. The quality of the filter — its pore sizes, charge, and surface area — determines what passes.
Anatomy of the glomerulus
The filter is a three-layered barrier plus supporting cells:
- Fenestrated endothelium (innermost):
- Endothelial cells have large pores (fenestrae) that allow plasma (but not cells) to approach the next layer.
- A glycocalyx (sugar-rich coating) lines these cells and helps repel negatively charged molecules (important for charge selectivity).
- Glomerular basement membrane (GBM):
- A dense extracellular matrix made of type IV collagen, laminin and heparan sulfate proteoglycans.
- Provides size and charge selectivity: its tight meshwork and negative charges hinder large and negatively charged proteins (like albumin).
- Podocytes and slit diaphragms (outermost):
- Podocytes are epithelial cells with foot processes that interlock, leaving narrow slit diaphragms between them.
- Slit diaphragms contain proteins such as nephrin and podocin that are structural and signaling molecules; damage here leads to major leakiness (proteinuria).
Between and beneath these layers are mesangial cells, which support the capillary loops, adjust surface area by contracting, and clear trapped debris.
The physics — Starling forces and the filtration equation
Fluid movement across a semipermeable membrane is driven by opposing forces: hydrostatic pressure (push) and oncotic pressure (pull due to proteins). The generalized Starling equation describes fluid flux (Jv):
Jv = Kf × [ (Pc - Pi) - σ(πc - πi) ]
Where:
Jv= fluid flux (volume/time) — positive when filtration occurs.Kf= filtration coefficient = surface area × hydraulic permeability (how leaky the membrane is to water).Pc= capillary hydrostatic pressure (push from inside the capillary).Pi= interstitial (or Bowman’s space) hydrostatic pressure (push back).πc= capillary oncotic (colloid osmotic) pressure (pull of plasma proteins).πi= interstitial oncotic pressure (usually small in Bowman’s space).σ= reflection coefficient (0–1) describing how well the membrane stops proteins (1 = perfect reflection; 0 = freely permeable).
Net filtration pressure is (Pc - Pi) - σ(πc - πi). Multiply that by Kf and you get how much plasma is filtered per minute. In the kidney, Kf is large (many capillary loops, a very permeable endothelium), and Pc is high because the glomerular capillaries are fed and drained by arterioles — a setup that favors filtration.
Quick plain-language summary: fluid moves out when the pressure pushing it out of the capillary exceeds the pressure pulling it back in (proteins) plus any resisting pressure in Bowman’s space.
Why the glomerulus favors filtration (and how it differs from ordinary capillaries)
- High capillary hydrostatic pressure (Pc): glomerular capillaries are “high-pressure” because both ends are arterioles.
- Low initial oncotic pull in Bowman’s space (πi is ~0): there are almost no proteins in initial filtrate, so there’s little pull back.
- Large Kf: huge surface area and permeability.
These factors make the glomerulus a very efficient ultrafilter — tuned to make a lot of plasma-derived fluid while retaining most proteins and cells.
Molecular players and biochemistry
- Albumin and other plasma proteins create capillary oncotic pressure (
πc). Loss of albumin (hypoalbuminemia) lowers πc and increases net filtration, contributing to edema in nephrotic states. - Heparan sulfate proteoglycans in the GBM provide negative charge; loss or modification (e.g., in diabetes or immune injury) reduces charge selectivity and lets albumin through.
- Nephrin/podocin: slit-diaphragm proteins that maintain the physical barrier and signal to the podocyte cytoskeleton. Podocyte injury (effacement) disrupts the diaphragms and causes heavy proteinuria.
- Endothelial glycocalyx: an underappreciated layer that repels proteins and contributes to the oncotic gradient; damage (in sepsis, hyperglycemia) increases leakiness.
- Mesangial cell signaling: angiotensin II, immune mediators and local growth factors can change mesangial tone and matrix, altering Kf.
Regulation of ultrafiltration (how GFR is controlled)
GFR (glomerular filtration rate) is essentially Kf × net filtration pressure. The body modulates filtration by changing any component:
- Afferent arteriole tone: constriction lowers Pc and reduces GFR; dilation increases GFR. Mediators include sympathetic nerves, prostaglandins (dilate), and NO.
- Efferent arteriole tone: mild constriction (e.g., by angiotensin II) raises Pc and increases GFR; marked constriction eventually reduces renal plasma flow and can lower GFR.
- Autoregulation: kidneys keep GFR steady over a wide blood pressure range via:
- Myogenic response: vascular smooth muscle contracts when stretched.
- Tubuloglomerular feedback: macula densa cells sense NaCl at the distal tubule and signal afferent arteriole tone — high NaCl → afferent constriction → lower GFR.
- Mesangial contraction: reduces available surface area (lowers Kf).
- Hormonal control: ANP increases GFR by relaxing mesangium and widening capillaries; angiotensin II preferentially constricts efferent arteriole.
Clinical pathophysiology — what happens when ultrafiltration goes wrong?
- Proteinuria / nephrotic syndrome: Damage to GBM or podocytes (e.g., minimal change disease, diabetic nephropathy, membranous nephropathy) increases permeability to albumin → albumin loss, hypoalbuminemia → decreased πc → more interstitial fluid accumulation (edema).
- Nephritic syndromes / glomerulonephritis: inflammation thickens or occludes capillary loops, reduces Kf, increases Bowman’s space pressure, and thus lowers GFR → hematuria, reduced filtration.
- Diabetic hyperfiltration: early diabetes produces efferent constriction and glomerular hypertension → increased Pc and filtration that eventually damages the filter, leading to proteinuria and progressive decline in function.
- Hypoalbuminemia (non-renal causes): lowers πc and can drive edema even without primary kidney disease.
- Systemic hypertension: sustained high pressures injure the glomerular capillaries → sclerosis and reduced filtration area (lower Kf).
Ultrafiltration beyond the glomerulus
- In systemic capillaries, the same Starling principles apply: arterial ends tend to filter, venous ends tend to reabsorb. But much of the filtered fluid is returned to circulation via lymphatics, not just reabsorption. The glycocalyx and interstitial oncotic pressure complicate the classic view; modern physiology recognizes continuous filtration and lymph flow as essential.
- In hemodialysis, “ultrafiltration” refers to using transmembrane pressure across the dialysis membrane to remove excess fluid from blood deliberately. Dialyzers have a Kf-like property and UF rate is controlled to prevent hypotension.
Practical measurements and tests
- GFR is the clinical measure of ultrafiltration efficiency. Gold-standard measurement uses inulin clearance; creatinine clearance (or serum creatinine-derived eGFR) is the common clinical proxy. Changes in GFR reflect changes in net filtration pressure and/or Kf.
Analogies that cement the concept
- Coffee filter: water and flavor pass, grounds stay.
- Sponge and sieve: sponge (interstitium/lymph) soaks up what the sieve (capillary) lets through.
- Garden hose with a nozzle: arteriolar tone changes the pressure inside the “hose” and therefore how much water sprays out the nozzle (filtrate).
Key takeaways (bullet form)
- Ultrafiltration = movement of plasma water and small solutes across a semipermeable membrane driven by hydrostatic/oncotic forces.
- In the kidney, a specialized 3-layer barrier (endothelium + GBM + podocytes) plus a high-pressure capillary layout makes for efficient filtration.
- The Starling equation (
Jv = Kf[(Pc - Pi) - σ(πc - πi)]) tells you what controls filtration: pressure push, protein pull, membrane area/permeability, and protein reflection. - Regulation is accomplished by changing arteriolar tone, mesangial contractility (Kf), and tubular signals (tubuloglomerular feedback).
- Damage to the filter (podocytes, GBM, glycocalyx) or changes in oncotic pressure produce clinically important outcomes like proteinuria and edema.