Imagine walking into a massive library where every element of the universe has its own shelf — neatly organized, labeled, and connected to others in a meaningful way. That’s the Periodic Table — the grand catalog of everything that makes up our physical world. From the iron in your blood to the silicon in your phone, every atom finds its place here.
What Is the Periodic Table?
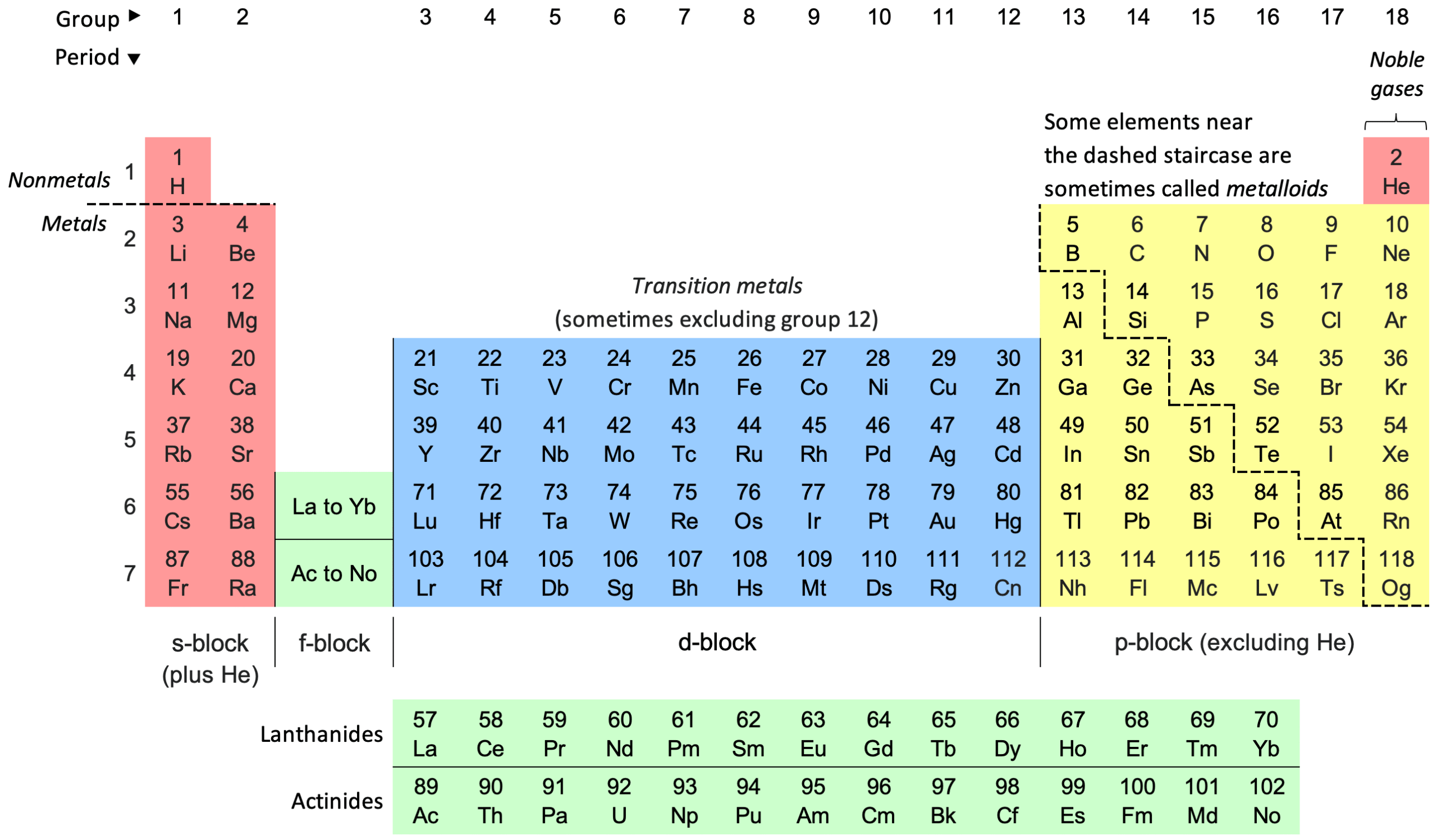
The Periodic Table is a scientific chart that arranges all known chemical elements according to their atomic number (the number of protons in their nucleus), electron configuration, and recurring chemical properties.
It’s not just a list — it’s a pattern, a map, and in many ways, a story of how matter behaves.
Each horizontal row is called a period, and each vertical column is a group or family. Elements in the same group often share similar properties — like relatives in the same family line.
How It All Started
In 1869, a Russian chemist named Dmitri Mendeleev noticed that when he arranged elements by their atomic weights, certain properties repeated periodically — like musical notes repeating after each octave.
He boldly left empty spaces, predicting elements that hadn’t yet been discovered. When those elements — like gallium and germanium — were later found, fitting exactly where he predicted, the Periodic Table became one of science’s greatest triumphs.
It proved something profound: there’s order beneath the chaos of matter.
The Order Behind the Table
Today, elements are arranged by atomic number instead of weight — because it’s the number of protons that defines what an element truly is.
For example:
- Hydrogen (1 proton) is the simplest element — the universe’s most abundant.
- Helium (2 protons) is what makes stars glow.
- Carbon (6 protons) builds the foundation of life itself.
- Iron (26 protons) fuels the hearts of stars and your blood.
The more protons, the heavier the element, and as you move across or down the table, the outer electron structure changes — giving rise to new chemical personalities.
The Magic of Periodicity
“Periodic” means repeating — and that’s the beauty of the table. Every few elements, patterns in behavior repeat because of how electrons fill up energy levels (or shells) around the nucleus. See Pauli Exclusion Principle, Aufbau’s Principle, Hund’s Rule.
Think of electrons like people filling seats in a theater — the first few rows (energy levels) fill up first. Once one row is full, the next begins.
The way these seats are filled determines how an element behaves — whether it’s reactive like sodium or stable like neon.
This repetition — or periodicity — gives rise to predictable trends:
- Atomic size decreases across a period (as protons pull electrons closer).
- Ionization energy increases (harder to remove an electron).
- Electronegativity — the pull of electrons — also strengthens.
These invisible trends are the grammar of chemistry — the rules that make matter make sense.
The Families of the Table
Each column has its own character:
- Group 1 (Alkali Metals): Soft, reactive, and love to lose an electron (like sodium and potassium).
- Group 2 (Alkaline Earth Metals): Slightly less reactive but vital to life (like calcium and magnesium).
- Group 17 (Halogens): Fierce electron lovers — reactive gases like fluorine and chlorine.
- Group 18 (Noble Gases): The calm ones — already stable, rarely reacting (like neon and argon).
Every element has a story, but together, they form a universal family portrait — the periodic rhythm of the cosmos.
Why It Matters
The Periodic Table isn’t just for chemists — it’s a map of reality.
It tells you:
- Why gold doesn’t rust.
- Why helium makes your voice funny.
- Why carbon can become either graphite or diamond.
- Why uranium can power cities.
It helps us predict, create, and understand everything — from medicine to materials, from stars to cells.
A Universe in a Table
Every square on that chart holds a universe within it — layers of electrons, forces, and energy. The periodic table is proof that nature is not random but beautifully ordered.
It’s humanity’s translation of the universe’s atomic language — where everything we touch, breathe, or build fits into one elegant system.
So next time you see it hanging on a classroom wall, don’t just see symbols and numbers — see the architecture of existence.
In Summary:
The Periodic Table is more than a chart — it’s the poetry of matter, written in symbols and numbers, sung by the universe itself.


Pingback: Pauli Exclusion Principle Simplified
Pingback: Electronic Configuration Explained Simply » Utopedia