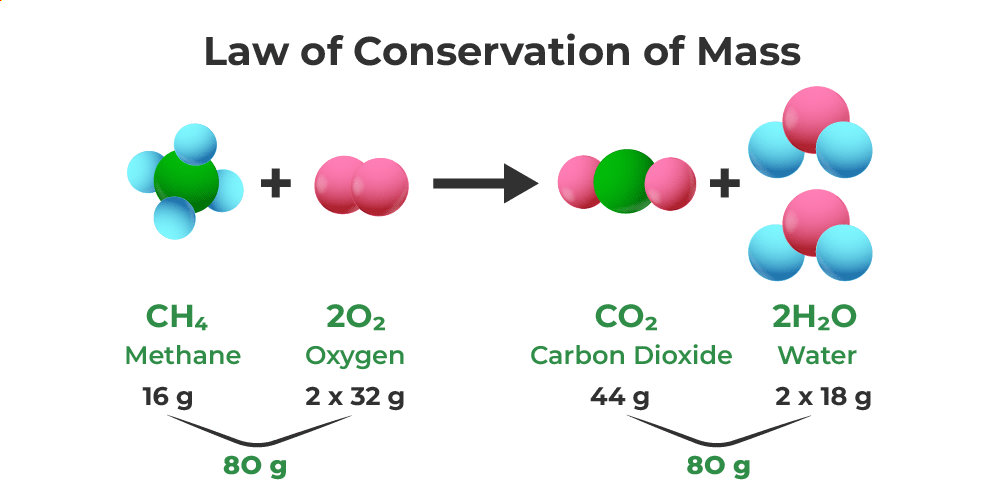What If Nothing in the Universe Could Ever Be Lost or Created?
Imagine a cosmic accountant so precise that every single atom is tracked throughout eternity—none can disappear, none can magically appear. This isn’t fantasy; it’s one of nature’s most fundamental rules: the Law of Conservation of Mass. Every chemical reaction, from the rust on your car to the food in your stomach, follows this unbreakable law.
The Definition
The Law of Conservation of Mass states that mass is neither created nor destroyed in chemical reactions. The total mass of reactants equals the total mass of products, meaning atoms are rearranged but never lost or gained during chemical processes.
To put it simply,
In any chemical reaction, you always end up with exactly the same amount of “stuff” you started with—it just gets rearranged into different forms. It’s like having 100 LEGO blocks that you can build into a car or a house, but you’ll always have exactly 100 blocks, never 99 or 101.
The Revolutionary Discovery
Before 1789, chemistry was basically educated guessing. Alchemists burned wood and watched it “disappear,” thinking mass was destroyed. They heated metals and saw them gain weight, believing mass was created from thin air.
Then Antoine Lavoisier, the “Father of Modern Chemistry,” conducted his famous sealed-container experiments. Here’s the shocking discovery: When he burned materials in completely sealed vessels, the total weight never changed! The wood seemed to disappear, but gases were produced with exactly the same mass. Metals gained weight by combining with oxygen from the air, which lost the same amount of mass.
Lavoisier’s revelation: Mass doesn’t vanish or appear—it just moves around and transforms.
Real-Life Examples
The Burning Candle Mystery
Light a candle and watch it “disappear.” Where did the wax go? It seems like mass vanished, but here’s what really happens: wax + oxygen → carbon dioxide + water vapor + heat. If you could capture all the invisible gases, they’d weigh exactly the same as the original wax plus the oxygen that was consumed. The mass didn’t disappear—it just became invisible gases floating away.
The Rusting Car Demonstration
Your car’s metal bumper gains weight when it rusts. Where does the extra mass come from? Iron + oxygen from air → iron oxide (rust). The “extra” weight comes from oxygen molecules that attached to the iron. It’s like iron atoms “eating” oxygen atoms—the total mass of iron + oxygen before equals the mass of rust after.
The Perfect Kitchen Scale
Imagine baking bread: flour + water + yeast → bread + steam + carbon dioxide. If you could weigh everything in a sealed oven (including all the steam and gas), the total would be identical before and after baking. The ingredients transform into bread and gases, but every gram is accounted for.
The Digestion Factory
When you eat an apple, it doesn’t just “disappear.” Apple + oxygen (from breathing) → carbon dioxide (exhaled) + water (sweated/exhaled) + energy + waste. Your body is a chemical factory that perfectly conserves mass—every atom from that apple ends up somewhere specific.
Supporting Scientific Principles
Atomic Theory (Dalton): Atoms are indivisible building blocks that can’t be created or destroyed in chemical reactions—only rearranged. Like permanent LEGO blocks that can build different structures but never change their essential nature.
Law of Definite Proportions: Compounds always contain the same elements in fixed ratios, supporting mass conservation by ensuring predictable, balanced reactions.
Avogadro’s Law: Equal volumes of gases contain equal numbers of particles, providing a way to count atoms and molecules to verify mass conservation.
Simplified Calculations
The Mass Balance Equation
Mass of Reactants = Mass of Products
Example Problem: Hydrogen burns in oxygen to make water
- Before: 4 grams hydrogen + 32 grams oxygen = 36 grams total
- After: 36 grams water
- Perfect balance: 36 grams in = 36 grams out
Step-by-Step Mass Tracking
- List all reactants (starting materials) and their masses
- List all products (ending materials) and their masses
- Add up each side and verify they’re equal
- If not equal, look for missing gases or products
Historical Experiments That Proved It
Lavoisier’s Sealed Mercury Experiment (1789)
He heated mercury in a sealed container for 12 days. Result: total mass unchanged, but some mercury became mercury oxide while oxygen disappeared from the air—perfect mass balance.
Modern Mass Spectrometry
Today’s instruments can track individual atoms through reactions, confirming that every single atom present before a reaction is still there afterward, just in different arrangements.
Common Misconceptions Busted
“Burning destroys mass” Wrong! Burning converts solid/liquid materials into invisible gases. A burning log becomes carbon dioxide, water vapor, and ash—same total mass, different forms.
“Digestion makes food disappear” Your body is a chemical processing plant. Food mass becomes new body tissue, energy (which has mass equivalent), waste products, and exhaled gases—everything adds up perfectly.
“Nuclear reactions violate conservation of mass” In nuclear reactions, tiny amounts of mass convert to energy (E=mc²), but the total mass-energy remains constant—it’s conservation of mass-energy, not just mass.
“Photosynthesis creates mass from sunlight” Plants don’t create mass from light energy. They use carbon dioxide from air + water from soil + light energy → glucose + oxygen. The mass comes from absorbed CO₂ and H₂O, not from sunlight.
Real-World Applications
Industrial Chemistry: Chemical plants use mass conservation to calculate exact amounts of raw materials needed and predict waste products—essential for cost control and environmental compliance.
Forensic Science: Investigators use mass balance to detect if evidence has been tampered with or to track the source of materials.
Environmental Science: Tracking pollutants through ecosystems relies on mass conservation—every atom of a contaminant can be followed from source to final destination.
Medicine: Drug dosage calculations depend on mass conservation to predict how medications are processed and eliminated from the body.
Why This Matters
The Law of Conservation of Mass isn’t just a chemistry classroom rule—it’s nature’s fundamental accounting system. It enables:
- Precise manufacturing of everything from medicines to smartphones
- Environmental protection by tracking pollutants
- Space exploration through exact fuel calculations
- Medical treatments with predictable drug interactions
The Bottom Line: Nothing in the universe ever truly disappears or magically appears. Every atom in your body was once part of ancient stars, will someday be part of future plants or animals, and is currently being perfectly tracked by nature’s cosmic bookkeeper. Mass conservation makes chemistry predictable, manufacturing possible, and the universe comprehensible.
From ancient alchemists’ confusion to modern precision manufacturing—understanding mass conservation transformed humanity’s ability to manipulate matter and harness nature’s power.