What If Water Could Act Like Molecular Scissors?
Every time you digest food, clean with soap, or even cry, water is performing one of chemistry’s most important tricks: breaking apart molecules by inserting itself into chemical bonds. This process, called hydrolysis, is so fundamental that life itself depends on it. But here’s the mind-blowing part—water doesn’t just dissolve things; it literally cuts molecular bonds and becomes part of the products. It’s like a molecular locksmith that picks locks by becoming part of the key.
The Science Definition
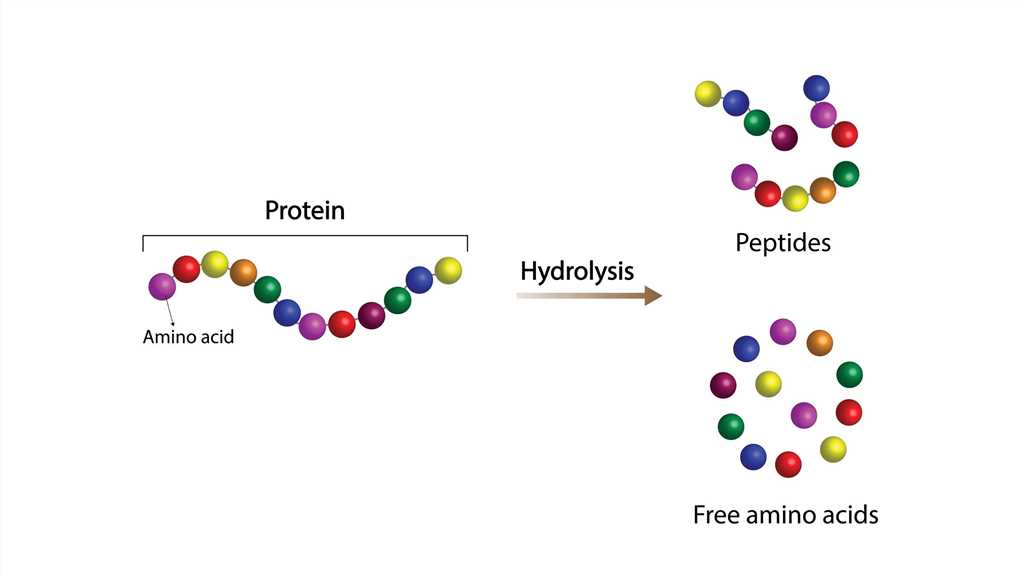
Hydrolysis is a chemical reaction in which water (H₂O) breaks chemical bonds by donating a hydrogen ion (H⁺) to one fragment of a molecule and a hydroxyl group (OH⁻) to another fragment, effectively cleaving the original molecule into two or more products. The water molecule is consumed in the reaction, with its components becoming integral parts of the resulting compounds.
In Plain English,
Hydrolysis is water’s way of breaking apart molecules by splitting itself in half and giving each piece to the fragments of the broken molecule. Think of it like a divorce mediator who solves the problem by marrying each of the separating parties. Water breaks up chemical marriages by becoming part of each new single molecule.
The Breaking Process
The Three-Step Dance
- Recognition: Water molecule approaches a vulnerable chemical bond
- Insertion: Water wedges itself into the bond, causing it to weaken
- Cleavage: The bond breaks, with H⁺ attaching to one fragment and OH⁻ to the other
The remarkable part: The original water molecule no longer exists—it has become part of two new molecules.
Types of Hydrolysis
Acid Hydrolysis
Water teams up with acids (extra H⁺ ions) to break bonds more aggressively. It’s like having a crowbar to help with the molecular breaking.
Example: Your stomach uses hydrochloric acid to help water break down proteins into amino acids during digestion.
Base Hydrolysis
Water works with bases (extra OH⁻ ions) to break different types of bonds. This is how soap works—it uses basic conditions to help water break apart greasy molecules.
Example: Soap making involves base hydrolysis of fats, literally breaking them apart and reforming them into soap molecules.
Enzymatic Hydrolysis
Special proteins (enzymes) guide water to break specific bonds with surgical precision. It’s like having a molecular GPS that directs water exactly where to cut.
Example: Digestive enzymes in your small intestine guide water to break starch into sugar molecules with incredible specificity.
The Familiar Examples
The Breakfast Breakdown Factory
When you eat toast, your saliva contains an enzyme (amylase) that guides water molecules to cut starch chains into smaller sugar pieces. Your mouth is literally a hydrolysis factory, with water molecules cutting up your breakfast before you even swallow.
The Chemistry of Tears
Your tears aren’t just saltwater—they contain enzymes that use hydrolysis to break down bacterial cell walls. When you cry, you’re essentially washing your eyes with natural molecular scissors that cut apart harmful microbes.
The Rust Revolution
When iron rusts, it’s partly due to hydrolysis. Water molecules break apart and attach to iron atoms, forming iron hydroxide. That reddish rust on your car is iron atoms that have been “married” to pieces of water molecules.
The Wood Rotting Reality
Fungi causing wood rot use hydrolysis to break down cellulose in wood. They release enzymes that guide water molecules to cut the long cellulose chains into smaller sugar molecules they can eat. Wood rot is essentially hydrolysis in slow motion.
The Detergent Detective Work
Laundry detergent works through hydrolysis. It helps water break apart the molecular “glue” that holds stains to fabric fibers. The detergent molecules act like molecular crowbars, helping water wedge into and break the bonds holding dirt to your clothes.
Biological Significance
Digestion: The Ultimate Breaking and Entering
- Proteins → Amino acids: Water breaks peptide bonds
- Carbohydrates → Simple sugars: Water cuts polysaccharide chains
- Fats → Fatty acids: Water breaks ester bonds in triglycerides
Cellular Energy Production
ATP (your cells’ energy currency) releases energy through hydrolysis. When your muscles need energy, water molecules break ATP bonds, releasing power and becoming part of the waste products.
DNA Repair
Your cells constantly use hydrolysis to remove damaged DNA sections and replace them with correct pieces. It’s like molecular editing, with water helping to cut out typos and insert corrections.
Simple Experiments You Can Try
The Starch Test
Mix cornstarch with water and add saliva. Wait 10 minutes, then test with iodine. The blue-black color (indicating starch) will fade as hydrolysis breaks starch into sugars.
The Fat Breaking Demo
Mix oil with water and soap. The soap uses hydrolysis to break oil molecules, allowing them to mix with water—demonstrating hydrolysis in your kitchen sink.
Let’s Bust Common Misconceptions
“Hydrolysis is just dissolving” Wrong! Dissolving moves molecules around; hydrolysis breaks them apart and creates new molecules. Sugar dissolving in water is different from starch being hydrolyzed into sugar.
“Water just helps reactions happen” Water is actually consumed in hydrolysis—it becomes part of the products. It’s not a bystander; it’s an active participant.
“Hydrolysis only happens with enzymes” While enzymes speed it up, hydrolysis can happen naturally. Given enough time, water alone can break many molecular bonds.
“All chemical reactions involving water are hydrolysis” Only reactions where water breaks bonds and becomes part of the products qualify as hydrolysis. Many water-involved reactions don’t break bonds.
Why This Matters
Food Processing: Hydrolysis breaks down proteins to create protein powders, converts starches to corn syrup, and produces artificial flavors.
Pharmaceuticals: Many drug manufacturing processes use controlled hydrolysis to break large molecules into active pharmaceutical ingredients.
Environmental Cleanup: Hydrolysis breaks down many pollutants naturally, which is why “biodegradable” products rely on water and enzymes to decompose safely.
Renewable Energy: Hydrolysis of biomass (breaking down plant material with water and enzymes) produces biofuels.
Why This Matters
Hydrolysis isn’t just chemistry textbook material. It’s the fundamental process that makes life possible. Every meal you digest, every cell repair your body makes, and every biodegradable product that safely decomposes relies on water’s ability to break molecular bonds.
Understanding hydrolysis helps explain everything from why certain materials biodegrade while others don’t, to how your body extracts energy from food, to how cleaning products work.
The Bottom Line: Hydrolysis reveals water as more than just a solvent—it’s nature’s universal molecular tool, capable of precisely breaking apart complex molecules and rebuilding them into simpler, useful forms. From the biochemistry of life to industrial manufacturing, hydrolysis is the invisible process that keeps our world running.
From ancient digestive processes to modern biotechnology—hydrolysis shows how water transforms from simple H₂O into the ultimate molecular multitool.


Pingback: Aluminum Ions: The Primary Cause of Soil Acidity » The Learning Hub