What If Every Recipe in the World Had to Follow Perfect Math?
Imagine baking cookies where using 2.1 eggs instead of exactly 2 would ruin the entire batch, or where doubling the recipe required precise mathematical calculations. Welcome to stoichiometry—chemistry’s version of cooking with recipes that must be followed to the molecule.
The Definition
Stoichiometry is the quantitative study of reactants and products in chemical reactions, based on the law of conservation of mass. It involves calculating the exact amounts of substances consumed and produced in reactions using balanced chemical equations and molar relationships, ensuring that atoms are neither created nor destroyed. To put it simply,
Stoichiometry is like following a super-precise recipe for chemical reactions. It tells you exactly how much of each ingredient (reactant) you need to make a specific amount of product, and guarantees that nothing gets wasted or magically appears—just perfectly balanced chemical cooking.
The Origin: From Alchemy to Precision
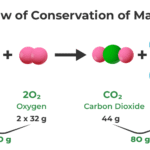
The term comes from Greek: “stoicheion” (element) and “metron” (measure). In 1792, German chemist Jeremias Richter first used it, but the real breakthrough came from Antoine Lavoisier’s discovery that “matter is neither created nor destroyed in chemical reactions”—the Law of Conservation of Mass.
Before this, chemistry was like cooking without measurements. Alchemists mixed random amounts hoping for gold. Lavoisier changed everything by introducing precise measurements, turning chemistry from medieval magic into modern science.
Real-Life Examples That Make It Click
The Perfect Sandwich Factory
Imagine a factory making ham sandwiches with this “recipe”: 2 bread slices + 1 ham slice = 1 sandwich. If you have 100 bread slices, you can make exactly 50 sandwiches (not 49, not 51). If you only have 30 ham slices but 100 bread slices, you’re “limited” by the ham—you can only make 30 sandwiches, leaving 40 bread slices unused. That leftover ingredient is called the “excess reactant”, while ham is your “limiting reactant”.
The Great Cake Baking Mystery
Your grandmother’s cake recipe calls for: 3 eggs + 2 cups flour + 1 cup sugar = 1 cake. If you want 5 cakes, stoichiometry tells you exactly what to buy: 15 eggs, 10 cups flour, 5 cups sugar. No guessing, no waste—perfect mathematical precision.
Rust Formation: Nature’s Stoichiometry
When iron rusts, it follows this reaction: 4 Fe (iron) + 3 O₂ (oxygen) → 2 Fe₂O₃ (rust). This means 4 iron atoms always react with exactly 3 oxygen molecules to produce 2 rust compounds. Nature never breaks this rule—it’s like having a cosmic recipe that’s always perfectly followed.
The Supporting Laws
Law of Conservation of Mass (Lavoisier): Matter cannot be created or destroyed, only rearranged. In our sandwich factory, you start with 100 bread + 50 ham pieces and end with 50 sandwiches—same total “stuff,” just reorganized.
Law of Definite Proportions (Proust): A compound always contains the same elements in the same proportions. Water is always H₂O—2 hydrogen atoms for every 1 oxygen atom, never 3:1 or 1:1.
Avogadro’s Law: Equal volumes of gases contain equal numbers of molecules under the same conditions—like having equal-sized boxes that always hold the same number of marbles.
Simplified Calculations
The Mole: Chemistry’s Dozen
Just as a dozen = 12 items, a mole = 6.02 × 10²³ particles (Avogadro’s number). Think of it as chemistry’s counting unit for incredibly tiny things.
Step-by-Step Stoichiometry
- Balance the equation (like writing the correct recipe)
- Convert to moles (translate ingredients to chemistry’s “dozen”)
- Use mole ratios (follow the recipe proportions)
- Convert back to desired units (get your final answer)
Example: How much water forms when hydrogen burns?
- Reaction: 2H₂ + O₂ → 2H₂O
- Translation: “2 hydrogen molecules + 1 oxygen molecule = 2 water molecules”
- If you start with 4 hydrogen molecules, you get exactly 4 water molecules
Real-World Applications
Pharmaceutical Manufacturing: Drug companies use stoichiometry to ensure exact dosages. Too little = ineffective; too much = dangerous.
Environmental Engineering: Calculating how much limestone neutralizes acid rain, or how much catalyst removes car exhaust pollutants.
Food Industry: Industrial baking requires perfect stoichiometry to ensure consistent taste, texture, and shelf life across millions of products.
Space Exploration: NASA calculates exact fuel mixtures for rocket engines—stoichiometric precision literally determines life or death in space.
Common Misconceptions Busted
“More reactant always gives more product” Wrong! Once you run out of your limiting reactant, the reaction stops—like running out of ham in your sandwich factory. Extra bread won’t make more sandwiches.
“Chemical equations are just suggestions” Nature doesn’t negotiate! The ratios in balanced equations are absolute laws. You cannot get 3 water molecules from the reaction that only allows 2.
“Stoichiometry is just academic math” Every manufactured product around you—from your phone’s battery to your morning coffee—exists because someone calculated stoichiometry correctly.
The Experimental Proof
Lavoisier’s Combustion Experiments: He showed that burning materials in closed containers always resulted in the same total mass before and after—proving matter conservation.
Modern Mass Spectrometry: Scientists can now count individual atoms and molecules, confirming that chemical reactions always follow exact mathematical ratios.
Why This Matters
Stoichiometry isn’t just chemistry class torture—it’s the foundation of our modern world. Every medicine you take, every material in your smartphone, every gallon of gasoline in your car exists because chemists and engineers apply stoichiometric principles to transform raw materials into useful products.
The Bottom Line: Stoichiometry is nature’s accounting system—a perfect mathematical balance where every atom is tracked, every reaction is predictable, and waste is minimized. It’s the difference between medieval alchemy and modern chemistry, between guessing and knowing, between chaos and precision.
From ancient alchemists’ wild experiments to today’s precision manufacturing—stoichiometry transformed chemistry from art to science.