Every atom is like a miniature solar system.
At its center lies the nucleus, dense and powerful.
Orbiting it are electrons — tiny particles moving in organized layers called shells or energy levels.
But they don’t move randomly.
Their arrangement follows a precise, elegant pattern — a code called electronic configuration.
It’s the rulebook of how electrons fill the space around an atom, shaping its behavior, bonding, and identity.
🔹 What Is Electronic Configuration?
In simple terms:
Electronic configuration describes how electrons are arranged in the shells and subshells of an atom.
Each electron has a specific address — like a home in a layered city.
And that address determines how it interacts with other atoms.
The Atomic “Neighborhoods”
Electrons don’t just orbit randomly — they occupy specific energy levels (shells), and within those shells, smaller subshells labeled as s, p, d, and f.
Here’s how it looks:
| Shell | Subshells | Max Electrons | Example |
|---|---|---|---|
| 1st (K) | 1s | 2 | Hydrogen (1s¹), Helium (1s²) |
| 2nd (L) | 2s, 2p | 8 | Oxygen (1s² 2s² 2p⁴) |
| 3rd (M) | 3s, 3p, 3d | 18 | Iron (1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁶ 4s²) |
| 4th (N) | 4s, 4p, 4d, 4f | 32 | Krypton (1s² … 4p⁶) |
Each subshell has orbitals, and each orbital holds up to 2 electrons spinning in opposite directions — a rule born from the Pauli Exclusion Principle.
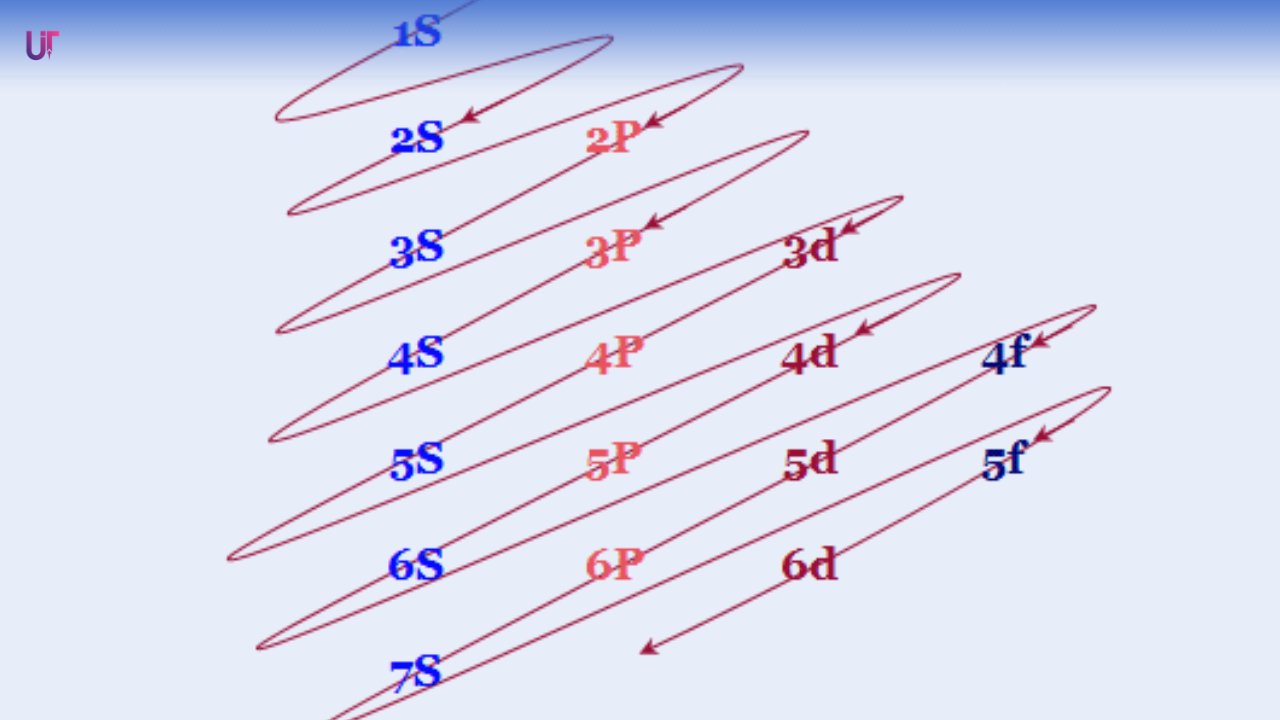
🔢 The Filling Order (Aufbau Principle)
Electrons fill the lowest energy levels first before moving upward — like students filling seats from the front row to the back.
This order follows the Aufbau principle (German for “building up”):
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d → 5p → 6s → 4f → 5d → 6p → 7s …
It looks complicated, but it’s nature’s way of staying efficient — lower energy first, higher energy later.
🔄 Hund’s Rule and Pauli’s Principle
Two key rules ensure that electrons fill up properly:
- Pauli Exclusion Principle:
No two electrons can have the same quantum state — each must differ by at least one property (like spin). - Hund’s Rule:
Electrons prefer to fill empty orbitals singly before pairing up — like passengers choosing their own seats on a bus before sitting next to someone.
Together, these rules make the electron arrangement stable and symmetrical.
Why It Matters
The electronic configuration isn’t just about numbers — it’s the blueprint of chemistry.
It determines:
- How an atom reacts (its valence electrons).
- How it bonds with others (ionic, covalent, metallic).
- Its position on the periodic table.
- Its magnetism, color, and conductivity.
For instance:
- Sodium (Na): 1s² 2s² 2p⁶ 3s¹ → easily loses one electron → highly reactive metal.
- Neon (Ne): 1s² 2s² 2p⁶ → stable and unreactive → noble gas.
So, electronic configuration explains chemical behavior at its core.
🧩 Simple Analogy
Think of a skyscraper with floors (shells) and rooms (orbitals).
Each floor has a certain number of rooms, and every room can house up to two people (electrons).
People (electrons) fill the lowest floors first — it’s easier and safer.
Once full, they move up to the next floor.
That’s exactly how nature fills energy levels — from low to high, following order and balance.
🪶 Simple Definition
Electronic configuration is the organized arrangement of electrons in an atom’s shells and subshells, determining its chemical properties and stability.
It’s how nature encodes atomic identity — a pattern that repeats beautifully across the periodic table.
In Summary
| Concept | Description |
|---|---|
| Definition | Arrangement of electrons in shells/subshells |
| Rule | Electrons fill lowest energy levels first |
| Governed by | Aufbau principle, Hund’s rule, Pauli principle |
| Importance | Defines chemical behavior and atomic structure |
| Example | Oxygen: 1s² 2s² 2p⁴ |
Final Thought
Every element in the universe — from gold to oxygen — follows this same pattern of order.
From the smallest atom to the heaviest metal, each electron knows exactly where to be.
Electronic configuration is the quiet rhythm of the atomic world —
a map of how energy, order, and identity dance in perfect balance.
It’s not just structure — it’s the signature of existence.